|
|
|
| Home > Market Information > Regulations / Policies |
 |
|
| |
| The following provides an overview of the tariff and
regulatory requirements for U.S. food and agricultural exports to
Korea. You may click on any heading below to go directly to
that section. For additional information please see
our 2020 Food and Agricultural Import Regulations and
Standards Report or email
atoseoul@fas.usda.gov |
| |
- Tariffs
- Tariff-Rate Quotas (TRQ's)
- Maximum Residue Levels (MRL)
- Food Additives
- Sanitary and Phytosanitary Requirements
- Biotechnology
- Labeling
- Organics
- Regulatory Agencies in Korea
|
| |
| Tariffs |
The Korea Customs Service is responsible for
administering the Customs Act of Korea, including classification, customs
clearance, and tariff collection. With the implementation of the KORUS FTA, all agricultural products imported from the United States will be subjected to the lower or eliminated import duty under the KORUS FTA. On-line information on current tariff rates, tariff-rate quota, rules of origin and safeguard information for the KORUS FTA is available on the ATO website at KORUS FTA.
Korea has free trade agreements with a number of
countries including Chile, the European Free Trade Association (EFTA), the
European Union, Association of South East Asian Nations (ASEAN),
Singapore, Peru and India (CEPA). Under these agreements, Korean tariffs on
agricultural products from these countries may be reduced or
eliminated. Additional information on the provisions of these
FTA’s can be found on the Ministry of Foreign Affairs and Trade website
at: MOFAT FTA. |
| |
| Traiff-Rate Quotas (TRQ's) |
Korea established a tariff-rate quotas (TRQ’s) for a
number of agricultural products in the WTO Uruguay Round negotiations.
Under a TRQ, imports up to a predetermined volume are assessed a lower
duty. Beyond that volume, the tariff is higher.
In addition to its WTO commitments, Korea may expand these TRQ’s or open additional TRQ’s to provide duty-free or lower duty access for some commodities, particularly those that serve as inputs to Korean agricultural industries such as livestock and food processing. Among the products currently subject to TRQ’s are pork, milk powders, whey products, butter, potatoes, garlic, barley and malting barley, corn, potato starch, and peanuts. Detailed information for CY 2012 can be found in the following GAIN Report.
Under the KORUS FTA, Korea also provides additional country-specific TRQ’s under the free trade agreement. Updated information about the TRQ allocation by products and its current usage is updated on the Korea Customs Service website but is only available in Korean. The ATO website provides an English translation of this information at KORUS FTA.
|
| |
| Maximum Residue Levels |
Differences in permitted residue levels for
pesticides and veterinary drugs can sometimes be non-tariff barriers to
trade. Three government agencies - the Korea Food & Drug
Administration (KFDA), the Ministry for Food, Agriculture, Forestry and
Fisheries (MIFAFF) and the Ministry of Environment (MOE) - handle
pesticide related matters. KFDA regulates pesticide residues in
foodstuffs. MIFAFF is responsible for pesticide registration and MOE
is responsible for testing pesticide levels in water, soil and
agricultural products.
KFDA is responsible for regulating pesticide residues
in foodstuffs, in accordance with the maximum residue levels (MRLs) set in the Food Code. As of December 2011, KFDA has set MRLs for 425 pesticides in agricultural products and 67 pesticides in ginseng products. The Food Code also lists MRLs for 83 pesticides and 110 veterinary drugs in meat, fish, eggs and milk products.
The latest Food Code posted on KFDA website
provides most updated MRLs in Korean. In addition to the Food Code, KFDA has set the MRL
database for agricultural products in Korean with English subtitles on its
website at:http://fse.foodnara.go.kr/residue/pesticides/pesticides_info.jsp
The following may help to determine the applicable
MRL for a particular crop:
I. Chemicals listed in the Food Code (Chapter 2)
1. Korea’s MRL set on the particular crop (individual)
2. CODEX MRL set on the particular crop (individual)
3. Korea’s lowest MRL set on the same crop group (For
fruit, nuts & oilseeds, and vegetables, a small group classification
applies first: e.g. in the absence of MRLs on apples in the Food Code and CODEX, the lowest MRL
set on pome group applies first. If there is no MRL set on pome group, then the lowest MRL set on fruit applies.)
4. Korea’s lowest MRL set for the particular chemical (regardless crop group)
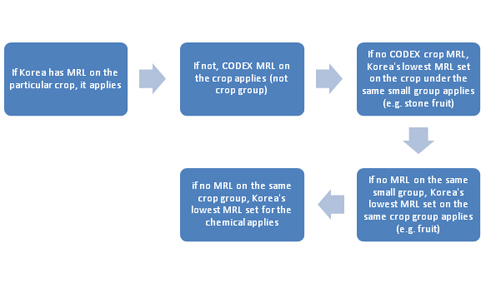
II. Chemicals not listed in the Food Code (Chapter 1)
1. CODEX MRL set on the particular crop (individual)
2. If CODEX does not have an individual MRL but a crop group MRL, then KFDA reviews the CODEX data package and determines acceptance of CODEX crop group MRL if the particular crop was included in the data package submitted to CODEX.
|
| |
Food Additives
|
The Food Additive Code guides the use of all additives in foods in Korea. As of December 2011, Korea had a positive list of 653 approved food additives and mixture of approved additives. Food additives are grouped into four categories: (a) chemical synthetics, (b) natural additives, (c) mixture substances, and (d) sanitizers.
Most additives and/or preservatives are approved and tolerance levels are established on a product-by-product basis in Korea. Getting a new additive added to the approved list usually takes a year or so. The “Guidelines for Designation of Food Additives” explains the detailed information required for the approval of a new additive.
The Food Additive Code may be found on KFDA’s English website:
http://fa.kfda.go.kr/foodadditivescode.html
|
| |
| Sanitary and Phytosanitary Requirements |
To prevent the introduction of pests and diseases, the Korean government requires sanitary or phytosanitary certificates issued by the exporting country’s inspection authority for importation of live animals, live plants, plant products including fruits and vegetables, and animal products including meat. This requirement is in accordance with the Livestock Epidemics Prevention & Control Act, the Plant
Protection Act, and the Act on Sanitary Control of Livestock Products.
For the United States, the U.S. Department of Agriculture (USDA), Animal Plant Health Inspection Service (APHIS), issues sanitary and phytosanitary certificates for live animals and plants, as well as plant and animal products, while the USDA, Food Safety Inspection
Service (FSIS), issues health certificates for meat products. Exporters should verify requirements for export to Korea before shipping.
|
Meat and Meat Products (Beef, pork, poultry):Current information on which U.S. livestock and poultry products are eligible for export to the Korean market can be found on the USDA, Food Safety Inspection Service (FSIS) website. This site also provides guidance regarding what documents must accompany livestock product shipments destined for Korea.
Special Requirements for Beef:Currently, MIFAFF only allows for the import of U.S. beef from cattle less than 30-months of age. The USDA Agricultural Marketing Service (AMS) Quality Systems Assessment (QSA) program verifies that the beef being certified is from cattle less than 30 months of age. At this time, Korea will not accept at port-of-entry shipments of beef without the QSA program statement in the Remarks section of the FSIS 9060-5 as described in the Documentation section. Korean quarantine officials will return shipments without the statement to the owner/agent of the product. A list of QSA approved establishments and their approval dates can be obtained from the AMS website.
In addition, Korea requires that beef imports come from plants approved under the Export Verification (EV) Program set up by USDA's Agricultural Marketing Service (AMS). Beef must be slaughtered and/or processed at plants listed in the Official Listing of Bovine Eligible Suppliers (aka, USDA Bovine EV Programs). This list can be obtained by visiting the following AMS Website:
http://www.ams.usda.gov/AMSv1.0/getfile?dDocName=STELPRD3105269
Beef that was slaughtered and processed at an EV program can be exported after being stored in a warehouse approved by USDA's Food Safety INspection Service. A list of all of the establishements on the Meat, Poultry and Egg Products Inspection Directory approved by FSIS for storing beef to be exported to Korea is available by visiting the following FSIS website. http://www.fsis.usda.gov/Regulations...
|
| |
| Biotechnology |
Imports of biotech grains as well as genetically engineered animals are regulated under the Living Modified Organism (LMO) Act, which implements the provisions of the Cartagena Protocol on Biosafety (CPB) and serves as the overarching legislation for regulation of living modified organisms.
Korea has two separate approval systems for biotechnology crops: approval for human consumption (a food safety approval)
and an environmental risk assessment (ERA). Both approvals are mandatory.
As of July 2012, KFDA has granted food safety approval to 77 events and RDA has approved 76 events for use in feed. (For labeling guidelines for genetically modified foods see “Labeling” section below.)
|
| |
| Labeling |
All imported food products are required to be labeled with the necessary information in Korean. Stickers may be used instead of
manufacturer-printed Korean language labels for imported food products. The sticker should not be easily removable and should not cover the original labeling.
Labeling standards for food products come under the authority of the Korean Food and Drug Administration (KFDA). KFDA regional offices inspect labeling of imported food products upon arrival. Provincial government health officials also have the authority to check labeling of both imported and
domestic products in the market place.
In general, labels should have the following information printed in letters large enough to be readily legible:
1. Product name
2. Product type: this is mandatory for specially designated products, such as teas, other beverages, extract products, special purpose foods, etc.
3. Importer's name and address, and the address where products may be returned or exchanged in the event of defects.
4. Manufacture date (date, month, and year): this is mandatory for specially designated products, such as boxed lunches, rice roll in seaweed, hamburgers, sandwiches, sugar, liquor (excluding beer and Korean traditional rice liquor since they are required to indicate shelf life), and salts. For liquors, a manufacture number (lot number) or bottling date can substitute for the manufacture date.
5. Shelf life or best before date: products including: jams, saccharide products (e.g. dextrin, oligosaccharide, and fructose), teas, coffee, sterilized beverages, bean based sauce and paste, sterilized curry products, vinegar, beer, starch, honey, wheat flour, etc. can use either a best before date or a shelf life date on the product label. If various kinds of products are packaged together, the shelf life expiration date of the product with the shortest life should be noted on the label.
6. Contents (Calories): calories are only required for food products subject to nutritional labeling.
7. Ingredient names and content.
8. Nutrients information: only designated products that are subject to nutritional labeling.
9. Other items designated by the detailed labeling standards for food. This includes cautions and standards for use or preservation (e.g., drained weight for canned products, radiation-processed products, etc.).
There are several categories exempted from the abovementioned labeling requirements including agricultural products such as grains; fishery items, such as whole frozen fish; and fruits, that are not contained in a container or package, etc. and food to be used for manufacturing for a company’s own use. In this case, limited information is required on the label. Appropriate documentation must be provided to verify end-use.
Labeling Guidelines for Genetically Modified Food:
Shipments consisting of 100 percent
unprocessed biotech crops for human consumption are required to carry labelsstating “GM ‘commodity’” (e.g. “GM soybeans”). Shipments that contain some biotech-enhanced crops are required to carry labels stating that the product “contains GM ‘commodity’” (e.g. “contains GM soybeans”). Shipments that may contain biotech-enhanced
crops are required to carry labels stating that the product “may contain GM ‘commodity’” (e.g. “may contain GM soybeans”).
Processed products containing biotech ingredients should be labeled as follows:
- Products that contain biotech crop or ingredients originated from biotech crops are required to be labeled as “GM food” or “food containing “GM commodity” (e.g. “GM corn or soybeans”).
- Products that may contain biotech crop are required to be labeled “May contain “GM commodity” (e.g. “May contain GM corn or soybeans”).
|
| |
| Organics |
Korea’s National Assembly passed the Ministry for Food, Agriculture, Forestry and Fisheries (MIFAFF)’s Act on Promotion of Eco-Friendly Agriculture and Management of Organic Products which includes organic certification. This new Act combines the organic regulations for fresh and processed products into one Act under MIFAFF. The Act will go into effect one year from promulgation, which is June 1, 2013.
In the meantime, Korea will maintain the existing two systems; one is recognition of imported organic products according to KFDA’s Labeling Standards for Food and MIFAFF’s certification system for both processed and non-processed organic products.
KFDA’s Labeling Standards for Food, which accepts processed organic products if they are certified by recognized foreign certifying agents such as NOP, will remain effective until December 31, 2012 at which time MIFAFF will assume full regulatory authority over organic products.
MIFAFF’s organic certification system is as below:
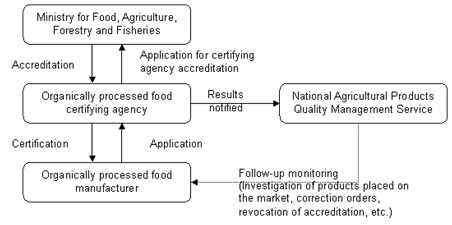
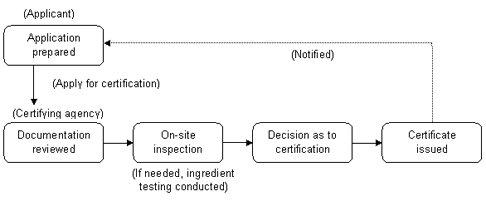
MIFAFF introduced a mandatory organic certification program for processed organic food products in June 2008. This program, which will be fully implemented starting January 1, 2013, will require all domestic and imported organic processed products to be certified by a MIFAFF-accredited certifying agent. MIFAFF’s accreditation and certification system will operate as shown in the flow diagrams below.
Accreditation Procedures for Organic Certifiers
Certification Procedures for Organic Producers
|
| |
Regulatory Agencies in Korea
|
Ministry of Health and Welfare:The Ministry of Health and Welfare (MHW) has responsibility for implementing the following legislation:
- Food Sanitation Act, is the legal basis for the food safety-related work conducted by MHW and the Korea Food & Drug Administration (KFDA)
- Functional Food Act, provides the legal basis for MHW and KFDA oversight of functional foods, such as health foods and nutritional supplements
- Special Act on Children’s Dietary Life Safety Management, :provides the legal basis for MHW and KFDA's determination and oversight of food products preferred by children. This Act restricts the sales and advertisements of high calorie low nutrient food products and introduces a voluntary color-coded labeling system.
Korea Food & Drug Administration:(KFDA) with its six regional offices is responsible for setting and enforcing standards and specifications for domestic and imported foods, functional foods, food additives, food packaging, containers and equipment. KFDA establishes the guidelines for implementing the Hazard Analysis of Critical Control Point (HACCP) program and recall systems for food products, excluding livestock and dairy products, which are regulated by MIFAFF. In addition, KFDA sets and implements regulations governing safety evaluations of agricultural products enhanced through biotechnology and labeling requirements for processed food products manufactured using GMO ingredients. Several of the key KFDA regulations are listed below.
- Food Code: stipulates standards and specifications for manufacturing, processing, usage, cooking, storage of food and equipment, containers and packaging for food products. It specifies the standards
for maximum residue levels of agricultural chemicals, antibiotics, synthetic antibiotics, hormones, radioactive ray standards, testing methods, etc.
- Food
Additive Code: defines standard specifications for individual food additives and usage standards.
- Labeling Standards for Food: provides guidance on how to meet KFDA’s Korean language labeling requirements for imported food products.
- Labeling Standards for Recombinant Food (GMO Labeling): provides standards required for labeling of processed food products containing GMO ingredients.
- Functional Food Code: contains general standards and specifications for functional foods
- Inspection Guidelines for Imported Food: checklist for imported food products detailing testing, sampling and other pertinent inspection standards.
Ministry for Food, Agriculture, Forestry and Fisheries(MIFAFF) is responsible for establishing regulations and standards related to agricultural products, including livestock and dairy products as well as forestry and fishery products. Several agencies within MIFAFF are responsible for issuing and enforcing regulations.
The Animal, Plant and Fisheries Quarantine and Inspection Agency (QIA) is responsible for establishing sanitary controls, standards, specifications and labeling requirements for domestic and imported livestock and dairy products in accordance with the Livestock Product Processing Control Act. QIA is responsible for HACCP and recalls for meat, poultry, eggs and dairy products.
The QIA is also responsible for preventing the introduction of harmful weeds, pests and disease originating from imported plants, fruits and vegetables. QIA conducts pest risk analysis, determines the appropriate eradication method for detected pests, and sets and enforces quarantine measures.
The Rural Development Administration (RDA) is responsible for developing the rural sector and administering policies on research and development, extension service, and training for farmers. RDA is pro-biotech and is actively pursuing GMO research in several food crops grown in Korea, such as virus resistant rice. RDA is the MIFAFF’s lead technical advisor on GMO-related policy. In addition, RDA conducts environmental risk assessments of biotech crops, in accordance with the LMO Act, which is the country’s enforcement legislation for the Cartagena Protocol on Biosafety.
The National Agricultural Product Quality Management Service (NAQS) is responsible for setting quality standards and grades for agricultural products, enforcing country of origin marks, GMO labeling requirements, and organic labeling for fresh fruits, vegetables, and grains in the marketplace, accrediting certifiers of non-processed organic produce, and post monitoring of labeling of organic processed food products in
the market place. NAQS collects samples from retail markets and tests products for GMO content with RDA-developed testing methods.
Several of the key MIFAFF regulations are listed below.
- Livestock Product Processing Control Act: specifies requirements for the slaughter and handling of livestock and the processing, distribution and inspection of livestock products.
- Livestock Code: provides health standards for meat, poultry and dairy products, such as microorganism standards, criteria and standards for livestock products, etc. (excluding MRLs for veterinary drugs and pesticide standards which are defined in the Food Code under the Food Sanitation Act)
- Labeling Standards for Livestock Products: provides the labeling standards for livestock products, containers, equipment, packaging and stamping dyes
- Import Health Requirements for Various Animals: provide health requirements for live animals and animal products. Korea’s health requirements for livestock and products can be found in English on the USDA’s Food Safety & Inspection Service (FSIS) website.
- Plant Protection Act: safeguards agricultural and forestry production by establishing quarantine regulations for imported and domestic plants.
- Import
Plant Inspection Guideline: defines inspection procedures for imported
plants and plant materials and establishes specific principles for the inspection
and disposition of imported plants.
- Agricultural
Products Quality Control Act: includes provisions governing agricultural
GMO products and labeling, country of origin marks, geographical
indication (GI), trace-back, etc.
- Guideline
for Labeling of Genetically Modified Agricultural Products: provides
details on labeling requirements for unprocessed GMO commodities,
including a list of commodities subject to GMO labeling, labeling methods,
etc.
- Guideline for Country of Origin (COO) for Agricultural Products
- Act on Promotion of Eco-Friendly Agriculture and Management of Organic Products: provides the legal basis for MIFAFF’s organic certification program and equivalence agreement.
|
Ministry of Knowledge Economy:(MKE) is the national competent authority for implementation of the Cartagena Protocol on Biosafety (CPB). Korea ratified the Cartagena Protocol on Biosafety (CPB) on October 2, 2007. Shortly thereafter, on January 1, 2008, Korea implemented the LMO Act, which is the implementing legislation for the CPB and the overarching law governing the country’s biotechnology related rules and regulations.
Several of the key MKE regulations are listed
below.
- Living Modified Organisms (LMO) Act: implements implements the Cartagena Protocol on Biosafety and to provide guidance on import approval, mandatory risk assessment, labeling, etc., of living modified organisms (LMO) or GMO commodities.
- Enforcement Decree and Rule of the LMO Act: stipulates the provisions delegated by the LMO Act
- Consolidated Notice: provides guidelines for export and import of LMOs for intended for agricultural use, intended for environmental release, intended for food, feed and processing and other use.
|
Prime Minister's Office:
Under the Framework Act on Food Safety, the Prime Minister’s Office is given the lead to coordinate the country’s food safety controls across the various ministries and agencies.
Under the
Act, each relevant agency was also tasked with developing a comprehensive 3-year food safety plan. In order to facilitate integration of these various plans, the law called for the establishment of a food safety committee with the Prime Minister serving as the chairperson. Committee members include: the Minister of Strategy and Finance, the Minister of Education, Science and Technology, the Minister of Justice, the Minister for Food, Agriculture, Forestry and Fisheries, the Minister of Health and Welfare, the Minister of Environment, the Commissioner of the Korea Food and Drug Administration, and Minister of the Prime Minister’s Office. |
| |
|